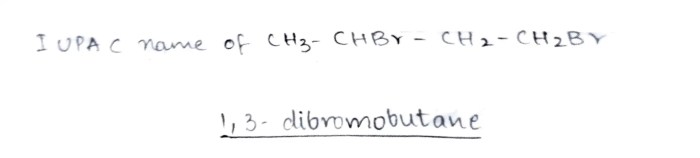
Heat of fusion and vaporization worksheet – Delving into the realm of heat of fusion and vaporization, this worksheet invites us on an intriguing journey to understand the energy dynamics governing phase transitions. These concepts, fundamental to chemistry and physics, underpin numerous applications in everyday life and industrial processes, making them essential knowledge for students and professionals alike.
Throughout this worksheet, we will explore the intricacies of heat of fusion, the energy required to transform a solid into a liquid, and heat of vaporization, the energy needed to convert a liquid into a gas. We will delve into the factors influencing these energy requirements, unravel the relationship between heat of fusion and vaporization, and uncover the significance of phase diagrams in representing these phenomena.
Heat of Fusion
Heat of fusion is the amount of energy required to change a solid substance into a liquid at its melting point. It is a measure of the strength of the intermolecular forces holding the molecules in the solid state. The heat of fusion is typically expressed in joules per mole (J/mol).
Examples of Heat of Fusion
- Water: 334 J/mol
- Ice: 601 J/mol
- Aluminum: 1079 J/mol
- Gold: 63 J/mol
Factors Affecting Heat of Fusion
The heat of fusion of a substance depends on several factors, including:
- Molecular structure: Substances with stronger intermolecular forces have higher heats of fusion.
- Molecular weight: Substances with higher molecular weights tend to have higher heats of fusion.
- Crystal structure: Substances with more ordered crystal structures have higher heats of fusion.
Heat of Vaporization
Heat of vaporization is the amount of energy required to change a liquid substance into a gas at its boiling point. It is a measure of the strength of the intermolecular forces holding the molecules in the liquid state. The heat of vaporization is typically expressed in joules per mole (J/mol).
Examples of Heat of Vaporization, Heat of fusion and vaporization worksheet
- Water: 2260 J/mol
- Ethanol: 38.6 kJ/mol
- Mercury: 59.2 kJ/mol
- Nitrogen: 5.56 kJ/mol
Factors Affecting Heat of Vaporization
The heat of vaporization of a substance depends on several factors, including:
- Molecular structure: Substances with stronger intermolecular forces have higher heats of vaporization.
- Molecular weight: Substances with higher molecular weights tend to have higher heats of vaporization.
- Temperature: The heat of vaporization decreases with increasing temperature.
Worksheet Problems
- Calculate the amount of heat required to melt 100 g of ice at 0°C.
- Calculate the amount of heat required to vaporize 50 g of water at 100°C.
Solutions
The heat of fusion of ice is 334 J/mol. The molar mass of water is 18 g/mol. Therefore, the amount of heat required to melt 100 g of ice is:“`(100 g / 18 g/mol)
334 J/mol = 1856 J
“`The heat of vaporization of water is 2260 J/mol. The molar mass of water is 18 g/mol. Therefore, the amount of heat required to vaporize 50 g of water is:“`(50 g / 18 g/mol)
2260 J/mol = 6278 J
“`
Applications: Heat Of Fusion And Vaporization Worksheet
Heat of fusion and heat of vaporization have numerous applications in everyday life and industry.
- Heating and cooling systems:Heat of fusion and heat of vaporization are used in heating and cooling systems, such as air conditioners and refrigerators, to transfer heat from one place to another.
- Food processing:Heat of fusion and heat of vaporization are used in food processing to preserve food by freezing and drying.
- Chemical industry:Heat of fusion and heat of vaporization are used in the chemical industry to separate and purify substances.
Advanced Concepts
Relationship between Heat of Fusion and Heat of Vaporization
The heat of fusion and heat of vaporization are related to each other by the Clausius-Clapeyron equation:“`ln(P2/P1) = (ΔHvap/R)
- (1/T1
- 1/T2)
“`where:* P1 and P2 are the vapor pressures at temperatures T1 and T2, respectively
- ΔHvap is the heat of vaporization
- R is the ideal gas constant
Phase Diagrams
Phase diagrams are graphical representations of the relationship between temperature, pressure, and the phases of a substance. They can be used to determine the heat of fusion and heat of vaporization of a substance.
Latent Heat
Latent heat is the heat that is absorbed or released by a substance during a phase change, such as melting or vaporization. It is a measure of the energy required to overcome the intermolecular forces holding the molecules in the solid or liquid state.
Top FAQs
What is the difference between heat of fusion and heat of vaporization?
Heat of fusion refers to the energy required to transform a solid into a liquid, while heat of vaporization refers to the energy needed to convert a liquid into a gas.
What factors influence the heat of fusion and vaporization of a substance?
Factors such as molecular structure, intermolecular forces, and temperature affect the heat of fusion and vaporization of a substance.
How are heat of fusion and heat of vaporization related?
Heat of fusion and heat of vaporization are related through the concept of latent heat, which represents the energy absorbed or released during a phase transition without a change in temperature.