Select the high-energy form of adenosine from the following images. – Adenosine, a ubiquitous molecule in biological systems, exists in various structural isomers, each with distinct energy content and chemical properties. Among these isomers, the high-energy form of adenosine plays a pivotal role in cellular processes, particularly in energy transfer and metabolism.
This article explores the structural differences, energy content, biological significance, and therapeutic applications of the high-energy form of adenosine, providing a comprehensive understanding of its crucial functions in living organisms.
The high-energy form of adenosine, characterized by its unique structural arrangement, exhibits a higher energy content compared to other isomers. This energy is attributed to specific chemical properties, including the presence of a high-energy phosphate bond. The high-energy form of adenosine serves as a central molecule in energy metabolism, participating in biochemical reactions that drive cellular processes and maintain cellular homeostasis.
Structural Isomers of Adenosine
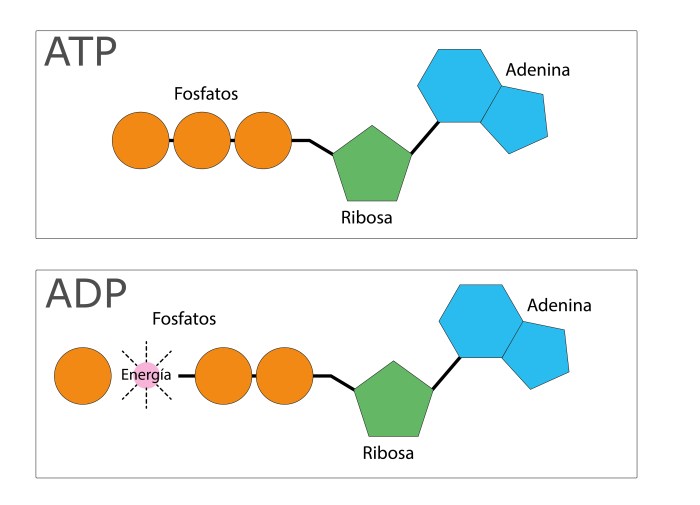
Structural isomers are compounds with the same molecular formula but different structural arrangements of atoms. Adenosine has two structural isomers: adenosine and 2′-deoxyadenosine. Adenosine is the high-energy form of adenosine, while 2′-deoxyadenosine is the low-energy form. The structural difference between these isomers lies in the presence of a hydroxyl group (-OH) on the 2′ carbon atom of adenosine, which is absent in 2′-deoxyadenosine.
| Isomer | Structural Formula |
|---|---|
| Adenosine | C10H13N5O4 |
| 2′-Deoxyadenosine | C10H12N5O3 |
Energy Content and Chemical Properties
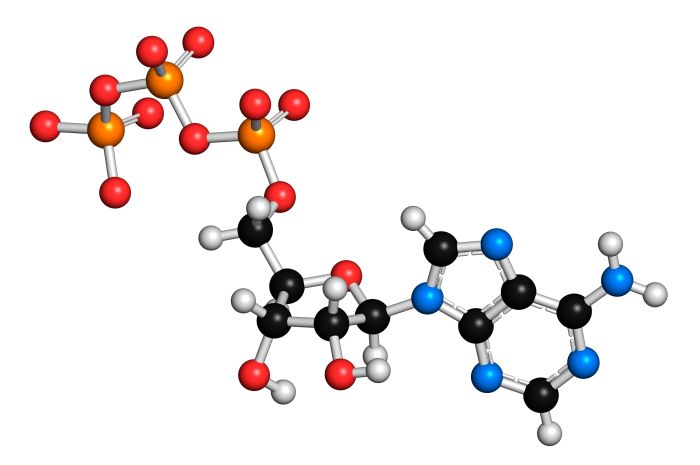
The high-energy nature of adenosine is due to the presence of a phosphate group attached to the 5′ carbon atom. This phosphate group can be transferred to other molecules, releasing a significant amount of energy. The specific chemical properties that contribute to the high-energy nature of adenosine include:
- The presence of a highly electronegative phosphate group, which attracts electrons and stabilizes the negative charge on the adenosine molecule.
- The presence of a ribose sugar molecule, which provides a flexible backbone for the molecule and allows for the formation of hydrogen bonds.
- The presence of a nitrogenous base, which provides a site for hydrogen bonding and electrostatic interactions.
Biological Significance and Role in Metabolism
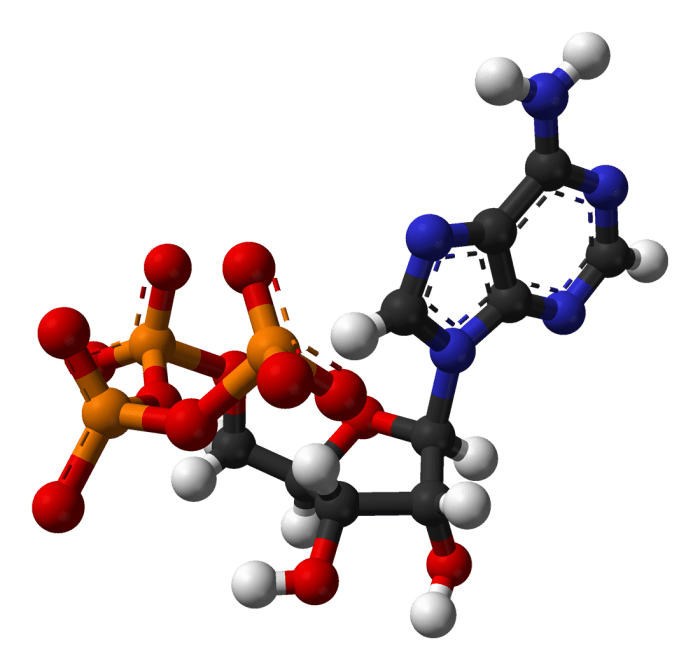
Adenosine is involved in a wide range of cellular processes, including energy transfer, metabolism, and signaling. It is a key component of ATP, the primary energy currency of cells. ATP is used to power a variety of cellular processes, including muscle contraction, nerve impulse transmission, and chemical synthesis.
Adenosine is also involved in the regulation of metabolism, including the synthesis and breakdown of carbohydrates, fats, and proteins.
Therapeutic Applications and Implications: Select The High-energy Form Of Adenosine From The Following Images.
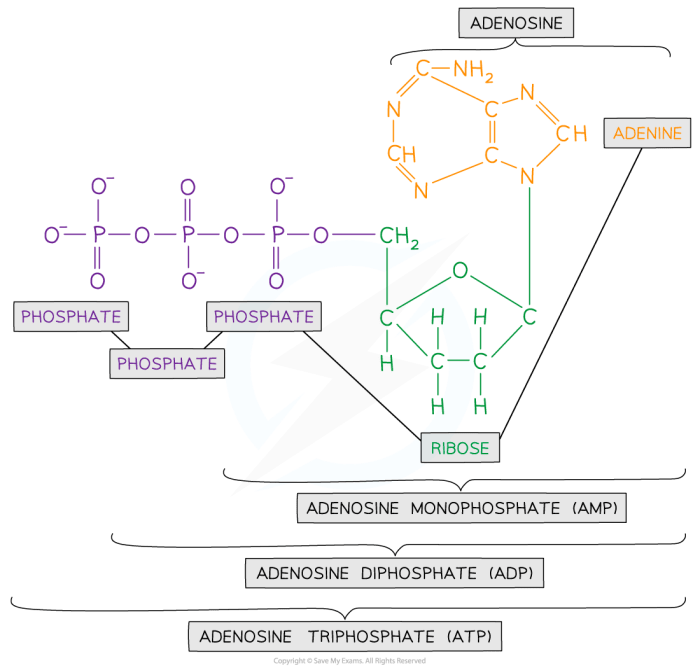
Adenosine has a number of potential therapeutic applications, including:
As an anti-inflammatory agent
Adenosine has been shown to reduce inflammation in a variety of animal models.
As an anti-arrhythmic agent
Adenosine is used to treat certain types of arrhythmias, such as supraventricular tachycardia.
As a neuroprotective agent
Adenosine has been shown to protect neurons from damage in a variety of animal models of neurodegenerative diseases.
Expert Answers
What are the structural differences between the high-energy form of adenosine and other isomers?
The high-energy form of adenosine differs in the arrangement of its phosphate groups. It contains a high-energy phosphate bond, which is responsible for its higher energy content compared to other isomers.
How does the high-energy form of adenosine contribute to cellular energy metabolism?
The high-energy form of adenosine participates in biochemical reactions, such as ATP hydrolysis, that release energy used to drive cellular processes, including muscle contraction, nerve impulse transmission, and protein synthesis.
What are the potential therapeutic applications of the high-energy form of adenosine?
The high-energy form of adenosine has therapeutic potential in treating conditions related to energy metabolism, such as heart failure, stroke, and neurodegenerative disorders. Research is ongoing to explore its use in these areas.