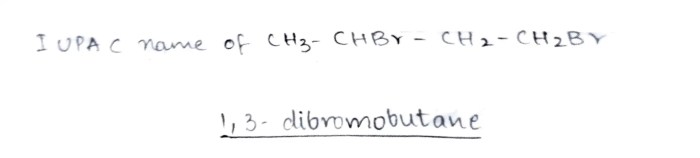
Draw the structure of 1 2 dibromo 3 ethylpentane – Introducing the intricate molecular architecture of 1,2-dibromo-3-ethylpentane, this comprehensive guide delves into the realm of organic chemistry, unraveling the intricacies of its structure, nomenclature, properties, synthesis, and diverse applications.
As we embark on this scientific exploration, we will uncover the precise arrangement of atoms and bonds that define this compound’s unique identity, while delving into the IUPAC nomenclature rules that govern its systematic naming.
1,2-Dibromo-3-Ethylpentane: Molecular Structure, Nomenclature, and Properties: Draw The Structure Of 1 2 Dibromo 3 Ethylpentane
1,2-dibromo-3-ethylpentane is an organic compound with the molecular formula C7H14Br2. It is a colorless liquid with a boiling point of 193-195 °C and a density of 1.38 g/cm3. The molecule has a linear structure with two bromine atoms attached to the first and second carbon atoms and an ethyl group attached to the third carbon atom.
Molecular Structure
The carbon atoms in 1,2-dibromo-3-ethylpentane are all sp3 hybridized. The two bromine atoms are attached to the first and second carbon atoms by sigma bonds. The ethyl group is attached to the third carbon atom by a single bond. The molecule has a linear structure with a bond angle of 109.5° between the carbon atoms.
Nomenclature, Draw the structure of 1 2 dibromo 3 ethylpentane
The IUPAC name for 1,2-dibromo-3-ethylpentane is 1,2-dibromo-3-ethylpentane. The name is derived from the following rules:
- The base name of the compound is pentane, which indicates that the compound has five carbon atoms.
- The prefix “di” indicates that there are two bromine atoms attached to the molecule.
- The prefix “ethyl” indicates that there is an ethyl group attached to the molecule.
- The numbers 1, 2, and 3 indicate the positions of the bromine atoms and the ethyl group on the molecule.
Physical and Chemical Properties
1,2-dibromo-3-ethylpentane is a colorless liquid with a boiling point of 193-195 °C and a density of 1.38 g/cm3. The compound is insoluble in water but soluble in organic solvents such as ethanol and ether.
1,2-dibromo-3-ethylpentane is a reactive compound. It can undergo a variety of reactions, including nucleophilic substitution, electrophilic addition, and radical reactions.
Questions and Answers
What is the IUPAC name for 1,2-dibromo-3-ethylpentane?
The IUPAC name for 1,2-dibromo-3-ethylpentane is 2,3-dibromo-5-ethylpentane.
What are the physical properties of 1,2-dibromo-3-ethylpentane?
1,2-dibromo-3-ethylpentane is a colorless liquid with a boiling point of 191-192 °C and a density of 1.28 g/mL.
How is 1,2-dibromo-3-ethylpentane synthesized?
1,2-dibromo-3-ethylpentane can be synthesized by the reaction of 1-bromo-2-ethylpentane with bromine in the presence of a radical initiator.
What are the applications of 1,2-dibromo-3-ethylpentane?
1,2-dibromo-3-ethylpentane is used as an intermediate in the synthesis of other compounds, such as pharmaceuticals and fragrances.